Imaging Technology Development
Q: How can we use real-time data to understand complex biology? There are lots of ways! You just need a good laser breadboard, some optics and a whole lot of patience. All of the images below were generated on microscopes that we built.
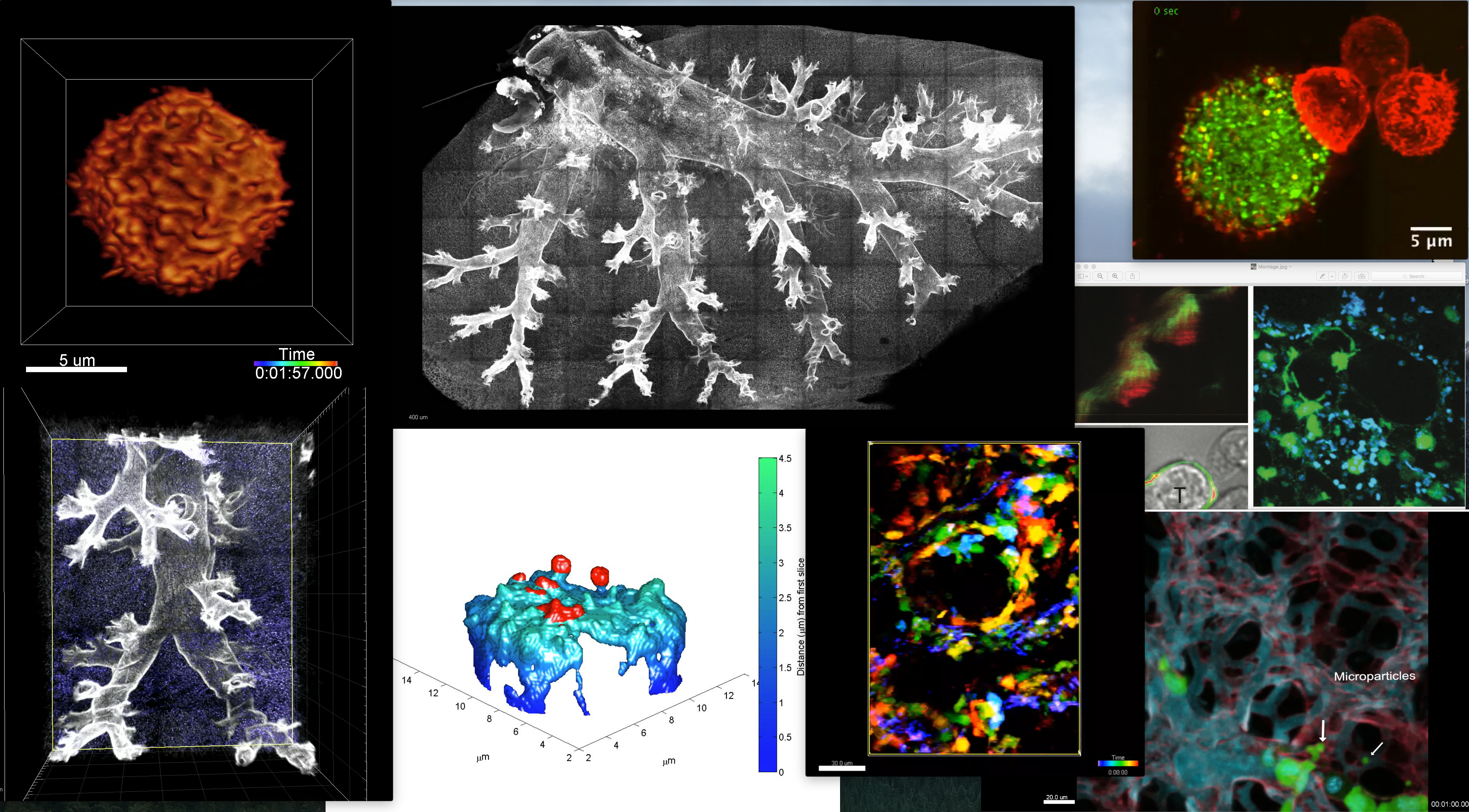
Current: Our lab has been active in developing and exploiting imaging technologies to understand how immune cells ‘talk to one another’ and make complex decision. Our recent efforts are in the area of spatial transcriptomics and in high-dimensional imaging of live biopsies. This builds on our successful builds of microscopes suited for high resolution imaging of synapses, microvilli and live tissues of organs. Read more about the collaboratory that arose from our lab here.
Historical: A significant advance for our work has been the use of 8- channel video-rate 2-photon microscopes (details at Bullen, A., Friedman, R.S., Krummel, M.F. Current Topics in Microbiology and Immunology. 2009) which allow a degree of spectral unmixing: to remove tissue autofluorescent signals. In one example, we used this method to overcome the low signal of an AIRE-GFP reporter in the context of the highly fluorescent lymph node capsule (Gardner et al. Science. 2008). This has also permitted the development of image-processing tools and methods to isolate TCR-GFP fluorescence from tissue autofluorescence so that the former can be studied in real-time in intact lymph nodes. (Friedman et al JEM 2010).
We’ve also developed imaging rigs for live-imaging lung (Looney et al Nature Methods 2011) and other intact organs.
As a companion to the optical technology, we’ve developed software for exploring complex tissues (Pinkard et al. Nature Methods 2016) as well as algorithms to improve signal detection arising from PMT-based multiphoton instruments (Pinkard et al.PlosOne, 2016). For those interested in multiphoton microscopes and in need of advice on how to choose one to suit their purposes, Kaitlin Corbin in the group wrote a terrific article on benchmarking these microscopes (Corbin et al. Methods in Current Biology, 2014).
More recently, in collaboration with Eric Betzig, we’ve built and adopted lattice light sheet imaging as a means to study T cell surfaces to understand how they ‘palpate’ and see their world (Cai et al. Science 2017). That study also pioneered a simple method, using Quantum dots and TIRF microscopy, to study synapse topology in real-time.
These advances continue to drive our research as we seek to develop and exploit novel biosensors in situ and detect relatively dim signals in the context of normal tissue and cells.
